Enhanced Generic HCP ELISA Kits

Host Cell Protein (HCP) monitoring during biological drug manufacturing is crucial. It is required to reduce the adverse side effects that the (high) abundance of host cell protein-derived impurities in the final drug substance may have when administered to humans, or when HCPs affect the drug product itself. Every individual HCP profile of a drug production is unique and depends on particular cell culturing conditions and the performance of manufacturing and downstream processes.
Our enhanced generic 360-HCP ELISA approach provides multiple kits, each with a unique set of anti-HCP antibodies, resulting in superior HCP assays that detect a broad range of HCPs. The ELISA kits are available for CHO, E. coli and HEK293 cell lines and suitable for early phases of drug and process development.
The Multi-Kit 360 HCP ELISA Approach for CHO, E. coli and HEK293
While traditional generic HCP assays provide just a single set of polyclonal antibodies towards one HCP antigen preparation that must work for all processes, the enhanced generic 360-HCP ELISA provides multiple kits, each using a different set of anti-HCP antibodies and/or HCP antigen materials.
Thus, the enhanced generic 360-HCP approach results in host cell protein (HCP) assays that are demonstrably superior to traditional generic HCP assays by minimizing well-known limitations. These include, for example, the possibility that single-kit anti-HCP antibodies might not comprehensively detect all HCPs present in a manufacturer's sample.
Learn more in our webinar “BioGenes' 360-HCP ELISA Kit Approach”
Generic ELISA Kit Generation
The manufacturing of each enhanced generic 360-HCP ELISA kit type involves the following steps:
- Immunization of different cohorts of animals and/or species using varying antigen material
- Affinity purification and production of each polyclonal antibody sets
- ELISA development, qualification and real time stability testing
- Manufacturing of ready-to-use ELISA kits
The antibodies of each individual kit type will display specific reactivity towards a subset of the total protein profile and will show weak or no reactivity towards other proteins of a given strain/ cell line. The proportion of proteins being recognized among a total population of proteins is termed coverage.
The superior 360-HCP ELISA approach uses Streptavidin-POD conjugate for the detection of biotin-labeled antibodies to ensure reproducible results. A two-step protocol was designed to to reduce nonspecific bindings and a standard stock solution is included for higher product stability and flexibility. The 360-HCP ELISA kits are optimized for automated plate washing to increase efficiency.
How to Select the Kit with the Best Fit?
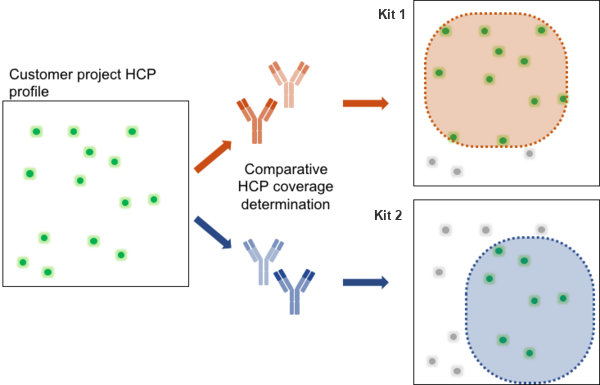
Each drug manufacturing process results in a particular HCP profile. To achieve optimal HCP monitoring, an evaluation study for best fit should be applied. As depicted, the antibody coverage of Kit 1 shows a broader range of HCPs in the exemplary project sample compared to Kit 2. Thus, Kit 1 would be the better choice to achieve monitoring of HCP reduction due to better overall coverage. For the identification of the generic 360 HCP ELISA kit with the best fit, BioGenes further recommends the evaluation of critical HCP ELISA performance criteria such as HCP coverage, sufficient HCP log-reduction, ELISA accuracy and sample dilution linearity.
Benefits
- Convenience: Our ready-to-use kits are readily available, saving you valuable time and effort in development.
- Cost-effectiveness: With our generic kits, you eliminate the need for expensive custom development, reducing your overall costs.
- Reliability: Our enhanced generic 360-HCP ELISA approach has been extensively qualified and proven to be superior to traditional generic HCP assays, ensuring accurate and reliable results.
- Comprehensive detection: The multiple anti-HCP antibody sets in our kits enable a more comprehensive detection of HCPs, minimizing the risk of missing impurities in process samples.