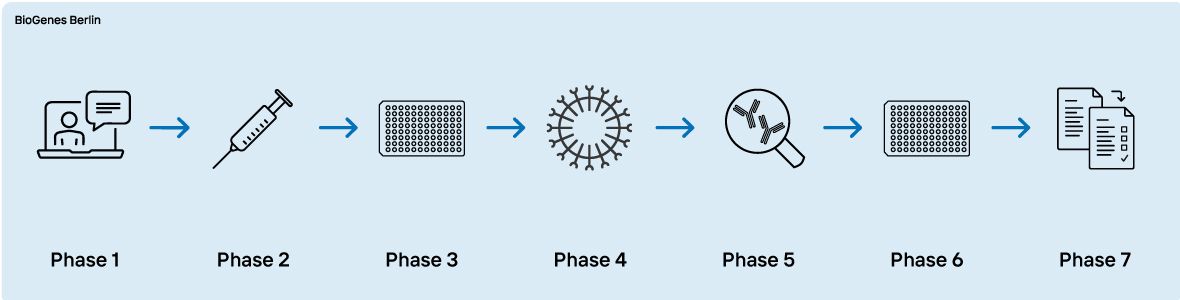
Phase 1 - Expert consultation
- Definition of the project´s aim
- Thourough discussion of antigen characteristics and project requirements

The assessment of drug efficacy and safety is of major concern in the pre-clinical and clinical testing phases of biologics drug development. As suggested by the “LADME” scheme, the distribution, metabolization and elimination of a pharmacologically active substance needs to be monitored. PK assays are used to determine the concentration of the drug in a relevant sample (e.g. patient serum) at given times. Regulatory authorities (the EMA and FDA) have released several guidelines for how these issues should be addressed using in vitro studies.
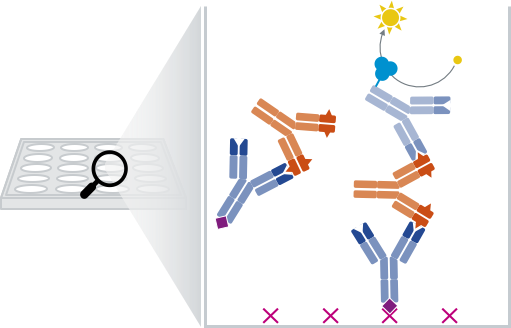
The sandwich ELISA is BioGenes' preferred approach of PK assays. This means that the therapeutic drug is successively captured and detected by two monoclonal antibodies, in a layered fashion.
Monoclonal anti-IDs are routinely used as capture and detecting reagents in pharmacokinetic (PK) assays for the quantification of antibody drugs in patient serum.
Our experts understand the difficulties and complexities that need to be addressed in your studies and will help you determine the optimal approach for PK and immunogenicity testing.
Phase 1 - Expert consultation
Phase 2 - Antigen preparation and immunization
Phase 3 - Cell fusion and hybridoma screening
Phase 4 - Cloning
Phase 5 - Pair search and antibody production
Phase 6 - ELISA development
Phase 7 - Method transfer
Approximate project duration: 12-18 months
To provide you with an all-inclusive service, BioGenes works closely together with FyoniBio. By combining our scientific expertise, we can offer you an all-in-one package ranging from custom antibody and assay development to ELISA validation and the analysis of pre-clinical and clinical samples according to GCLP guidelines. However, customers can also choose to independently obtain the individual services from either company alone.
Contact
If you need more information, a specific offer
or want to talk to an expert
Newsletter
Keep me updated