Analytical Services
The presence of host cell proteins (HCPs) in biologics must be strictly monitored and manufacturing processes need to be optimized to reduce impurities and their risks to a minimum. Profound sample characterization, in-depth analysis using orthogonal methods and facultative protein identification are key for successful drug manufacturing and approval by authorities such as the FDA and EMA.
BioGenes specializes in the use of two-dimensional Polyacrylamide Gel Electrophoresis (2D PAGE) for sample and reagent characterization by highly qualified staff and state-of-the-art equipment. BioGenes’ 2D methods include 2D Western Blot analysis of HCP coverage and 2D Difference Gel Electrophoresis (2D DIGE) for comparative analysis of sample spot patterns.
Moreover, we offer Immunoaffinity Chromatography (IAC) in combination with 2D DIGE as orthogonal method for HCP coverage determination and optional Mass Spectrometry (MS) for further protein identification.
The choice of methods for your project is individual. The scientific team of BioGenes will be happy to support you.
Method Overview
2D PAGE
Two-dimensional Polyacrylamide Gel Electrophoresis (2D PAGE) enables separation of complex protein samples by charge and size. BioGenes uses high-resolution instrumentation, including horizontal flat-bed SDS-PAGE, to enable maximal resolution of sample protein spots and superior visualization of low molecular weight protein species.
Comparison of different 2D PAGE resolutions


Advantages of 2D PAGE
- Visualization of sample proteins for characterizing intermediates of a downstream purification process and for monitoring HCP impurities
- Various staining methods including fluorescent minimal labeling (pre-labeling), Coomassie staining, Silver staining and sensitive fluorescent total protein staining (post-labeling)
- High resolution for improved protein visualization including low molecular weight proteins
- Spot picking for protein identification by MS
2D Western Blot
The assay setup for HCP investigation at BioGenes includes 2D Western Blot analysis, which is a widely accepted standard method for evaluating HCP antibody coverage. BioGenes mostly uses fluorescent minimal labeling for total protein visualization and fluorescent immunodetection with custom-made or generic anti-HCP antibodies and a suitable fluorophore-conjugated detector antibody. By doing so, total protein visualization and immunostaining can be performed on the same Western blot membrane with overlapping signals indicating successful coverage.


Advantages of 2D Western Blot
- Accepted standard method by regulatory authorities
- Low material usage
- Fluorescent multiplexing enables precise coverage analysis
- High sensitivity
- Direct interaction between HCPs and anti-HCP antibodies
- Enables coverage analysis of process samples dissolved in harsh buffers
2D DIGE
The generation of suitable antigens for HCP antibody development is crucial for the detection of all relevant HCPs of a real product-production process and the success of any given HCP ELISA project. 2D DIGE analysis is highly useful for comparing spot patterns of complex HCP samples and assessing similarity. BioGenes offers comparative 2D DIGE analysis of HCP populations. This technique is further used as a visual read-out for assessing HCP coverage after Immunoaffinity Chromatography (IAC). In addition to qualitative pattern comparison, 2D DIGE may also be used to assess changes in protein abundance between samples.
2D DIGE analysis of mock and process sample
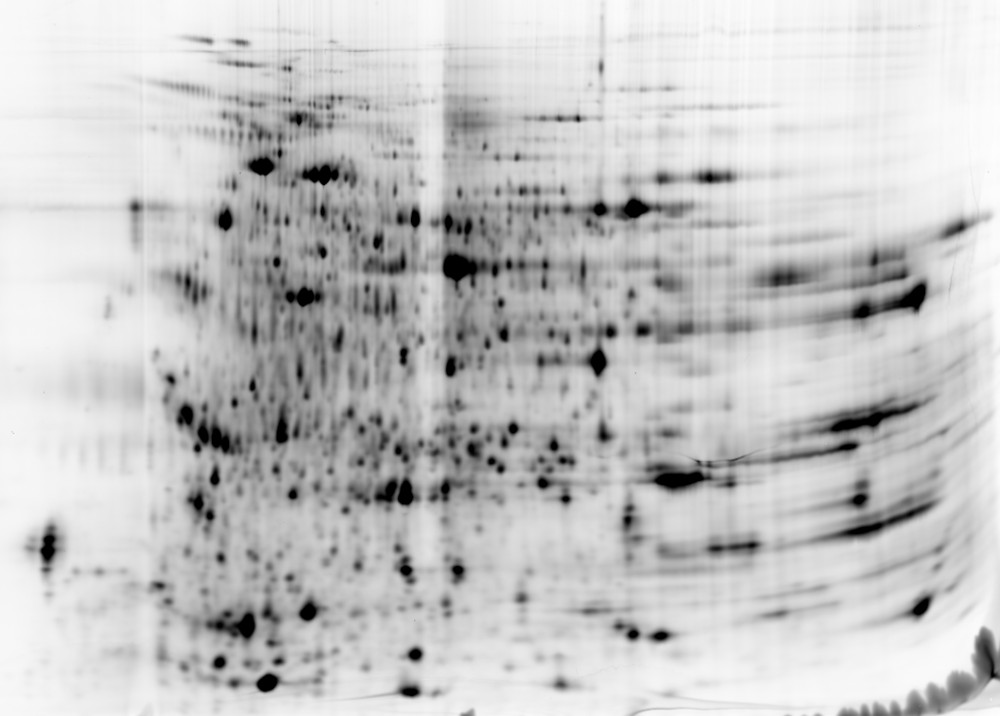

IAC + 2D DIGE
The traditional 2D Western blot method has been widely accepted for HCP coverage determination. However, it may also exhibit potential technical challenges, including denaturing assay conditions and a limited dynamic range. To overcome these challenges, BioGenes has optimized IAC in combination with 2D DIGE as an orthogonal method for coverage determination. IAC serves as a superior method enabling HCP binding to an immobilized capture antibody under nearly native assay conditions (close to ELISA). Following the chromatographic step, 2D DIGE is used to visualize and directly compare the HCP spectrum of the eluted sample fraction to that of the input sample.
Advantages of IAC+2D DIGE
- Non-denaturing method
- Higher dynamic range
- Improved suitability for product-containing samples
- Visual read-out
IAC + MS
MS-based coverage HCP-analysis is a highly valuable tool in the field of biopharmaceuticals, providing insights into the identity of host cell proteins. BioGenes offers comprehensive MS analysis of IAC input and elution fractions in collaboration with experienced partners.
- Highly customizable: Can be tailored to meet the specific needs of individual clients
- Suitable to determine coverage
- Insight into the identity of proteins in a sample
- Risk assessment of individual proteins
Protein Identification after 1D or 2D PAGE
For in-depth analysis of specific HCPs, we offer protein identification by MS methods after SDS PAGE. Following one- or two-dimensional protein separation, protein bands will be visualized using suitable total protein stains with high sensitivity. For target protein identification, protein extraction from bands of interest can be performed, followed by a proteolytic digest and subsequent MS analysis. This service will be provided in collaboration with our experienced partners.
Contact
If you need more information, a specific offer
or want to talk to an expert.
Newsletter
Keep me updated
